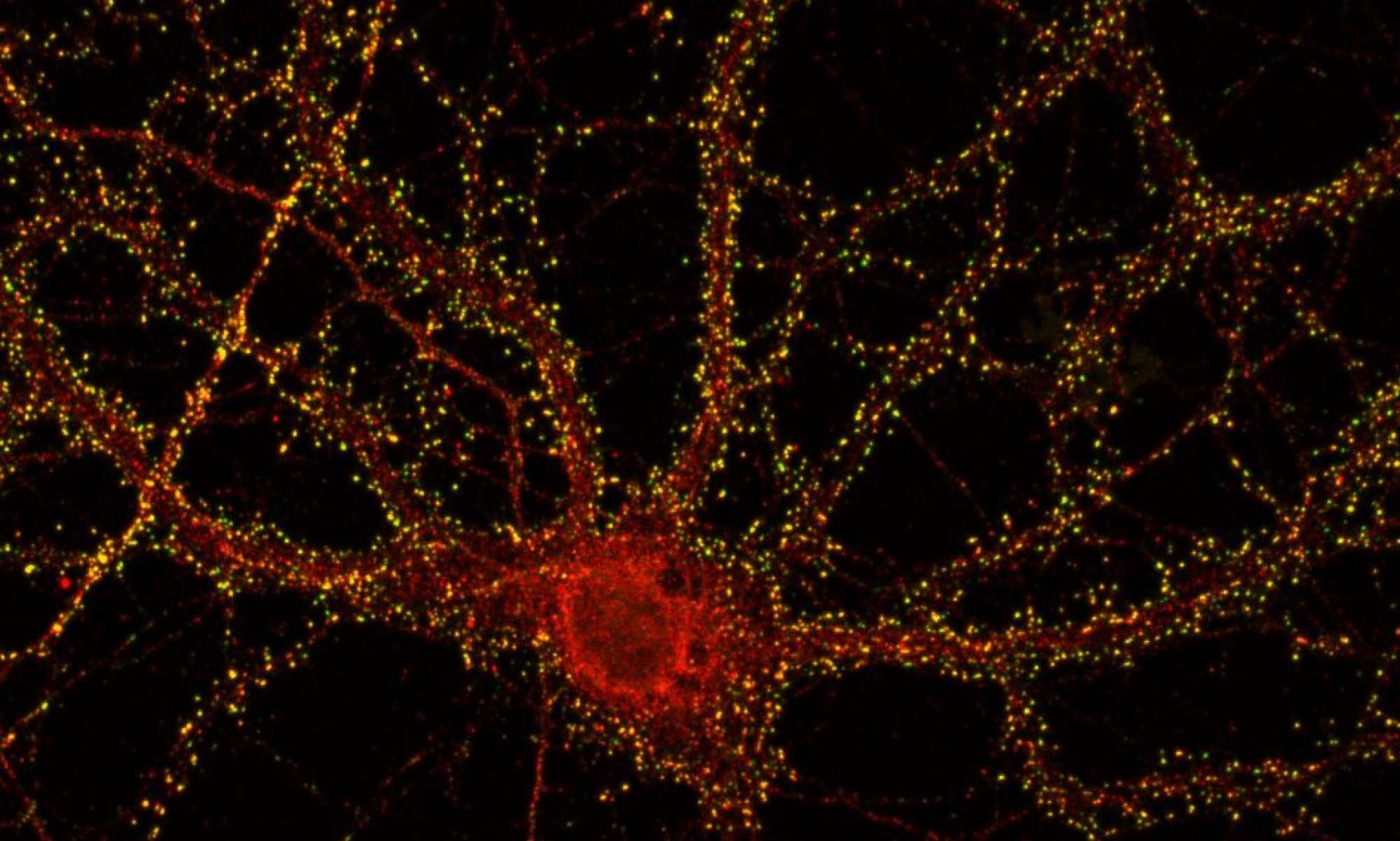
The lab is interested in molecular and cellular mechanisms that control plasticity of neurotransmitter release and their role in normal brain function and in disease.
Our publications on PubMed.
There are four main areas of research that we are currently working on:
Research Area 1: Molecular Mechanisms of Neurotransmitter Release
Using transgenic mice, gene deletion and replacement strategy, and imaging of key synaptic processes (glutamate release, calcium influx, synaptic vesicle fusion and retrieval), we investigate the functional contribution, interaction and regulation of molecular components of presynaptic release sites. These studies revealed the key function of the interplay between presynaptic cytomatrix and calcium channels in controlling neurotransmitter release (Davydova et al., 2014, Heck et al., 2019) or the link between presynaptic cytomatrix and endocytic machinery (Ivanova et al., 2020). We are continuing this line of research by investigating the mechanisms of presynaptic calcium homeostasis and the spatio-temporal coupling of exo- and endocytosis of synaptic vesicles.
Research Area 2: Modulation of Neurotransmitter Release in the Context of Disease
Taking advantage of our deep understanding of presynaptic physiology, we are investigating how disease-relevant compounds (drugs or physiological modulators) affect neurotransmitter release. We were able to demonstrate the effect of endogenously produced amyloid β, a peptide associated with Alzheimer’s disease, on the modulation of neurotransmission via positive allosteric modulation of the α7 nicotinic acetylcholine receptor (Lazarevic et al., 2017; Anni et al., 2021). We have also identified the same receptor as a mediator of the effect of hydroxynorketamine (the long-lived metabolite of the rapid antidepressant ketamine) on neurotransmitter release and neuronal gene expression (Guhathakurta et al., 2024).
Research Area 3: Cellular Signalling in the Presynaptic Compartment
Usage-dependent changes in the efficiency and mode of neurotransmitter release contribute to neuronal plasticity, the cellular underpinning of learning and memory. The molecular mechanisms underlying this process are incompletely understood. Our studies in this context revealed a novel PKA-cAMP-PDE4 signalling axis in the regulation of synaptic vesicle release and retrieval (Montenegro-Venegas et al., 2022). In addition, we uncovered presynapse-specific regulation of proteasomal activity that mechanistically contributes to neurodegeneration (Montenegro-Venegas et al., 2020; Schattling et al., 2019). We also described that dysregulation of RAS-MAPK signalling in RASopathies, a group of genetic diseases, significantly affects neurotransmission (Altmüller et al., 2017; Weiss et al., 2024). We proposed that RASopathies, which often present with neurological and psychiatric diagnoses, are developmental synaptopathies. We are following this line in the EURAS consortium, which was recently funded by the EU with more than €8 million.
Research Area 4: Synapse to Nucleus Signalling in the Context of Neuron-Glia Interaction
We have identified a novel synapse to nucleus signalling pathway that controls gene expression depending on neuronal activity and cellular metabolic status (Ivanova et al., 2015, 2016). Our current research indicates that this pathway acts in orchestrating neuron-glia metabolic interaction, which is important for efficient neurotransmission and neuronal plasticity, and for maintaining the neuron-astrocyte and neuron-oligodendrocyte functional unit.